Are you tired of baby pictures? This should hit the spot then.
The water in Bancroft tastes fine, though it has a ton of temporary hardness (the kind that drops out when water is boiled). Since beer is 95% water, the hardness does affect it. My beers in Nebraska have been held back by this water hardness, I believe.
This experiment tested a way to reduce the temporary hardness, without boiling (which is expensive for ~10 gallons). I used slaked or pickling lime (Calcium Hydroxide) in the water to precipitate Calcium Carbonate, which eliminates temporary hardness as Calcium Bicarbonate (CaCO3) at the bottom of the vessel.
The white stuff at the bottom is the temporary hardness precipitated out, after settling out over night.
I used four 5 gallon carboys to do the test. I used an aquarium alkalinity test measurement kit to get the results.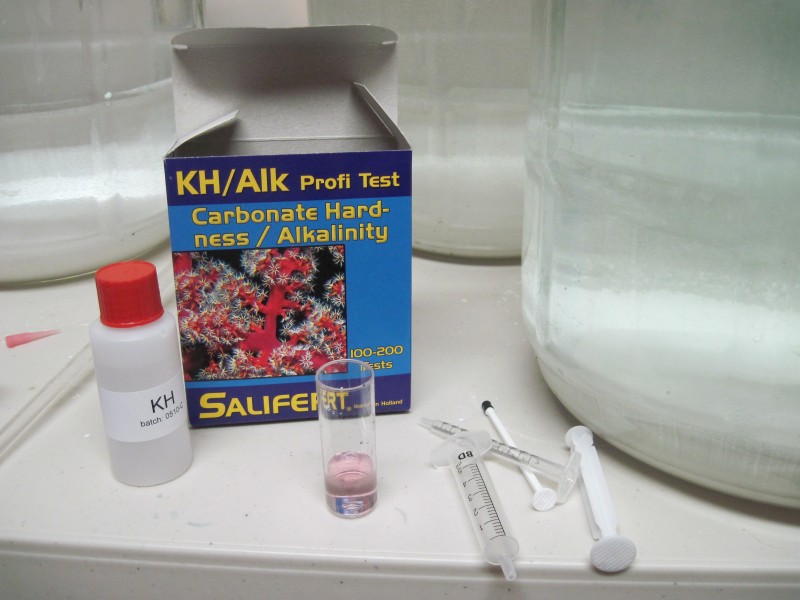
I thought it did the test wrong, but it turned out that the tap water is above the limit of the kit. But from the water report I know the temporary hardness is 320ppm. The kit only can measure to 285pmm CaCO3. My temporary hardness is too much for even beers requiring the darkest grains. It leads to all kinds of problems in light beers, as I have found out over the past several years (high mash ph, poor efficiency, astringency, and a harsh hop flavor).
1st carboy: 5 grams of slaked lime. The resulting harness was 170ppm CaCO3.
2nd carboy: 6g lime. Result: 130ppm CaCO3
3rd carboy: 6g lime + 1g Gypsum (CaSO4) + CaCL. Result: 65ppm. The limiting factor for this process is Calcium in the water. So adding Ca before allows more of the CO3 to combine with Ca and drop out. All Ca is removed unless there is a surplus of Ca. The formula is (I think) : CaCO3 precipitated = Ca * 2.5. I do not have enough naturally occurring Ca to remove all the bicarbonate.
4th carboy: 7g lime. Result: 145ppm CaCO3.
Conclusion
All the white stuff at the bottom demonstrated that it worked. Considering that I only put in 5-7 grams of lime, there was an lot of CaCO3 at the bottom of the carboys. The additional 2 grams of salt reduced the total alkalinity by 80%. The limit of this process according to others is 40-50ppm CaCO3. Since much if not all of the Calcium is removed, it will have to be added back in before the mash in the form of Gypsum (CaSO4) or Calcium Cloride (CaCl). But control over those elements in the water is desired anyway by the demanding brewer.
This process is cheap and fairly easy. It should help me brew better beer and is cheaper and more convenient than buying water from the store.
For more information see:

Really cool. It is amazing how much dropped out. I cant wait until next summer to try them, or maybe sooner 😉